One of the three major milestones of the GYM project is to conduct a phase I/II clinical study in which patient-own muscle stem cells will be systemically transplanted. Aims of this study is to determine the safety and effectiveness of intra-arterial delivery of patient-own muscle stem cells. This progress update will provide you with a bit of background on our journey so far and an update on the research that is currently ongoing research in preparation of the phase I/II clinical study within the GYM project.
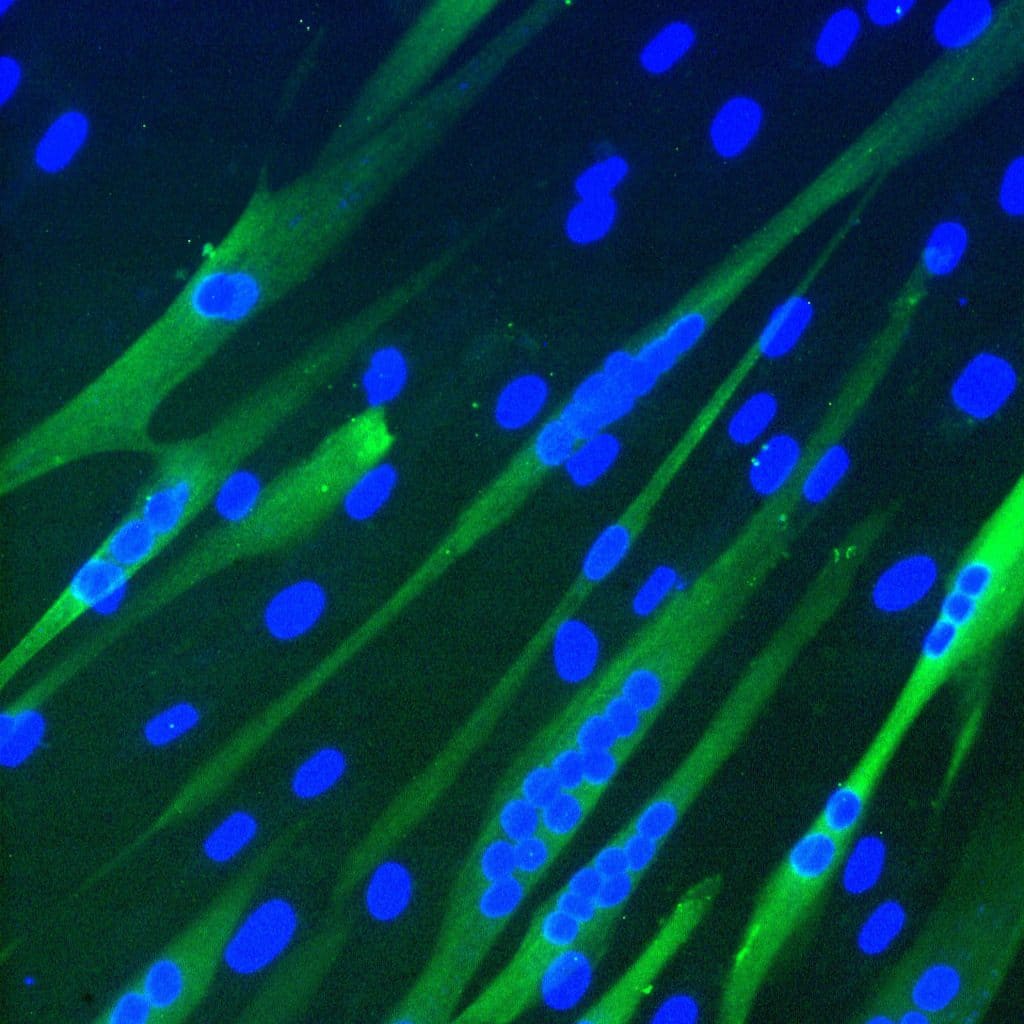
The muscle stem cells that we are using in this project are called mesoangioblasts (MABs) and these stem cells are located on the blood vessels. When isolated from a skeletal muscle biopsy, the MABs have a high myogenic capacity, in other words, they are very capable of forming new muscle fibers, (illustrated in the picture above of MABs-derived multinucleated muscle cells). Moreover, MABs can be injected into the blood stream and migrate to damaged muscle, herewith enabling systemic delivery and treatment of multiple muscle groups at once.
Improvement
Our collaborator Maurilio Sampaolesi demonstrated in 2006 that systemic injection of donor MABs can lead to functional improvement in a dog model of Duchene Muscular Dystrophy (DMD) , which led to the first in man clinical trial of donor mesoangioblasts in boys with DMD by Prof. Cossu et al.. Drawback of using donor MABs is the requirement for immunosuppressive agents and in order to circumvent this, we use patient-own MABs. However, this requires that the MABs do not contain a genetic defect. In 2012, we started analysing MABs from patients with a genetic defect in their mitochondrial DNA (mtDNA). A mutation in the mtDNA leads to impaired energy production by the mitochondria and has most impact on high energy-requiring tissues, like the muscles. In contrast to a nuclear DNA mutation that is present at equal amount in all cells, a mtDNA mutation is not present in equal amounts between tissues, and there can also be cells that are mtDNA mutation-free. We demonstrated that MABs of nearly half of the mtDNA mutation carriers were (nearly) mtDNA mutation free and that displayed normal growth, myogenesis and mitochondrial function (van Tienen et al.). Taken together, these MABs fulfilled all the criteria to be used for cell therapy to induce muscle formation.
Clinical study
In order to use cells as cell therapy medicinal product, they need to be cultured at a Good Manufacturing Process (GMP) facility, according to standard operating procedures and using only clinical grade reagents. This process has been developed and validated for culturing MABs in sufficient amounts for clinical application. Currently, the first clinical trial in which mtDNA mutation-free patient-own MABs are injected is currently ongoing at Maastricht UMC. This is the first in men clinical study and will mainly provide information with respect to the safety. In addition, we will also assess migration and local muscle-formation in the muscle of the lower leg.
Within the GYM project, our partner, the GMP facility at University of Liege, will culture GMP-grade mesoangioblasts and transfer and testing of the GMP culture procedures is currently ongoing. In addition, our partners from UHasselt are currently optimizing the exercise training required to stimulate migration of MABs from the bloodstream towards the effected muscles. The effect of this exercise bout will also be assess non-invasively using the MRI scanners of partner Scannexus. If successful, this will provide an objective and non-invasive readout to assess the muscle damage prior to transplantation. If you are interested in participating in this exercise training study, please check the website within a couple of weeks for more information.