pH and Thrombin Concentration Are Decisive in Synthesizing Stiff, Stable, and Open-Porous Fibrin-Collagen Hydrogel Blends without Chemical Cross-Linker
Abstract
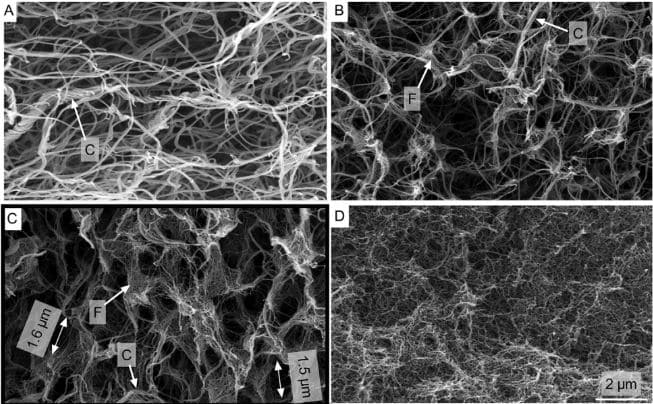
Fibrin-collagen hydrogel blends exhibit high potential for tissue engineering applications. However, it is still unclear whether the underlying cross-linking mechanisms are of chemical or physical nature. It is here hypothesized that chemical cross-linkers play a negligible role and that instead pH and thrombin concentration are decisive for synthetizing blends with high stiffness and hydrolytic stability. Different fibrin-collagen formulations (pure and with additional transglutaminase) are used and the blends’ compaction rate, hydrolytic stability, compressive strength, and hydrogel microstructure are investigated. The effect of thrombin concentration on gel compaction is examined and the importance of pH control during synthesis observed. It is revealed that transglutaminase impairs gel stability and it is deduced that fibrin-collagen blends mainly cross-link by mechanical interactions due to physical fibril entanglement as opposed to covalent bonds from chemical cross-linking. High thrombin concentrations and basic pH during synthesis reduce gel compaction and enhance stiffness and long-term stability. Scanning electron microscopy reveals a highly interpenetrating fibrous network with unique, interconnected open-porous microstructures. Endothelial cells proliferate on the blends and form a confluent monolayer. This study reveals the underlying cross-linking mechanisms and presents enhanced fibrin-collagen blends with high stiffness, hydrolytic stability, and large, interconnected pores; findings that offer high potential for advanced tissue engineering applications.